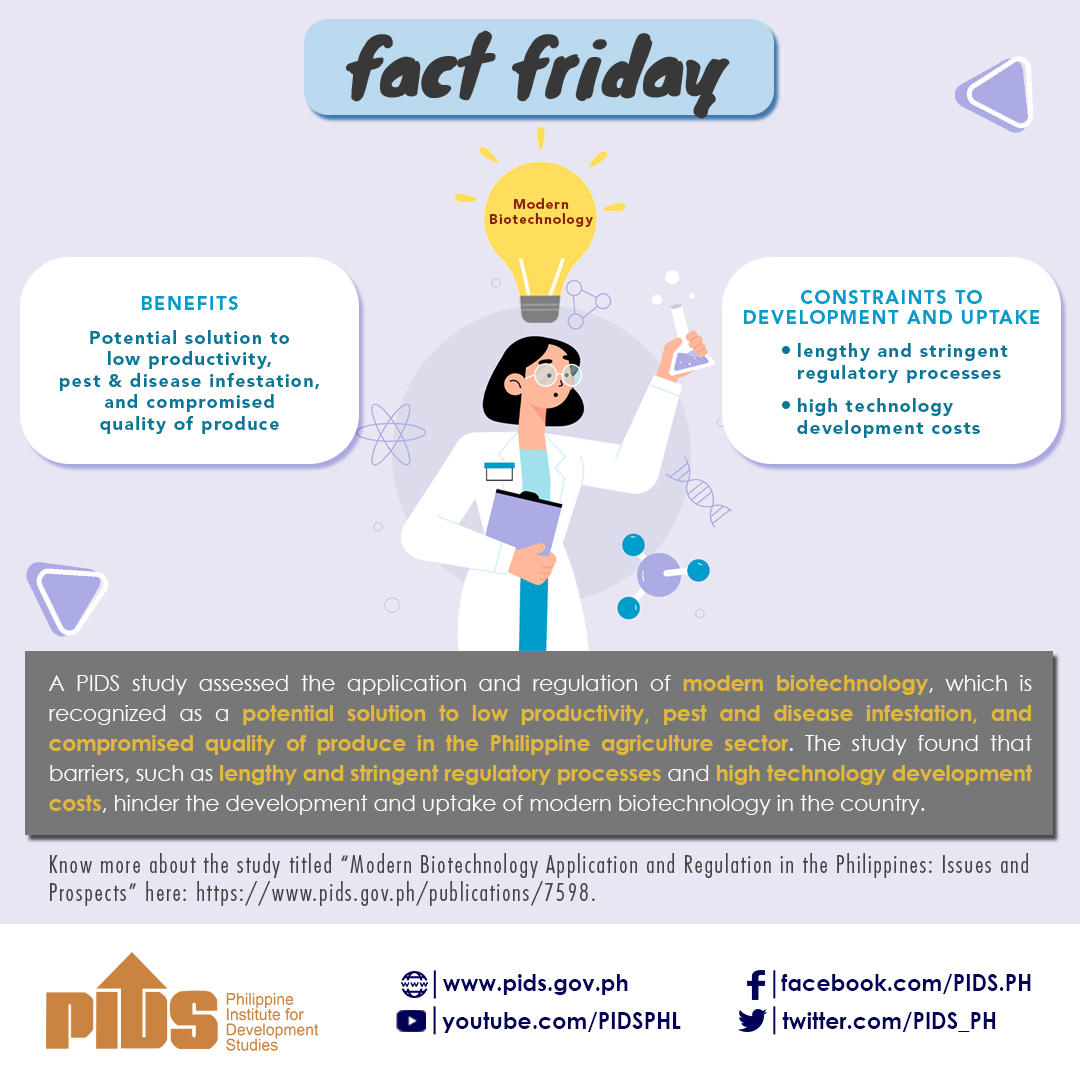
The existing regulations for products of modern biotechnology are ‘burdensome’.
This was one of the key points raised by Dr. Eufemio Rasco Jr., academician of the National Academy of Science and Technology, during a webinar recently organized by the Philippine Institute for Development Studies (PIDS) on modern biotechnology application and regulation in the country.
According to Rasco, the regulatory processes for modern biotechnology products are justified based on the assumption that modern biotechnology is “inherently risky”.
“The basic assumption that modern biotechnology is inherently risky is a product of ignorance of the scientific community in the 1980s when this technology was new and poorly understood,” he explained, adding that “mainstream science no longer subscribes to this view” after gaining “more recent knowledge”.
In addition, Rasco pointed out that modern biotechnology products can be regulated under existing laws that regulate introduction of food products, which include determining the safety of food products by “looking at the product itself, not the process by which they were created”.
“For new varieties of plants, for example, the regulatory system does not discriminate whether it was produced using conventional breeding, mutation breeding using chemicals or radiation, or through interspecific crosses. If there is a known risk of a toxin or allergen, the regulatory process requires toxicity or allergenicity studies,” Rasco explained.
The University of the Philippines Mindanao professor emeritus also highlighted the “relative safety” of modern biotechnology, as shown by “more than 25 years of farming experience over billions of hectares and consumption by billions of consumers”.
With this, Rasco emphasized the importance of a science-based regulatory regime for modern biotechnology products that is product based and not process based.
“There is no reason why products of modern biotechnology should be treated differently from other products of plant breeding,” he said.
Rasco also suggested conducting risk assessment on a case-by-case basis. According to him, this should be applied to all products of genetic modification, regardless if it is through modern biotechnology or conventional breeding.
Rasco likewise recommended the “repudiation” of the special regulatory regime for modern biotechnology products, pointing out that the regulations are “burdensome, not only to researchers, but also to government, which has to harness the services of seven national government departments to implement the regulations”.
Rasco also proposed for the withdrawal of support for the Cartagena Protocol on Biosafety, which according to him is the basis for Philippine regulation of modern biotechnology, because it is based on “outdated scientific assumption”.
Lastly, Rasco called for support on a bill that seeks to create a “biotechnology authority”.
“If we imagine the present regulations as brakes of a biotech car, the proposed bill will provide the accelerator and steering wheel. After all, we are not going anywhere in a car with only the brakes in it,” he concluded.